SALSA MC002 SMA Newborn Screen
The Melt Assay SALSA MC002 SMA Newborn Screen is an in vitro diagnostic (IVD)1 or research use only (RUO) semi-quantitative assay for the detection of homozygous deletions of exon 7 in the SMN1 gene in genomic DNA isolated from human peripheral whole blood specimens or dry blood spot (DBS) cards. MC002 SMA Newborn Screen is intended to confirm a potential cause for and clinical diagnosis of spinal muscular atrophy (SMA) and for molecular genetic screening of newborns. The MC002 assay cannot determine absolute SMN1 or SMN2 copy numbers with the exception of 0 copies.
In most populations, a homozygous loss of the SMN1 gene, usually detected by the absence of exon 7 specific markers, is the cause of disease in the majority of SMA patients (>95%)2. Homozygous SMN1 exon 7 deletions detected with MC002 SMA Newborn Screen should be confirmed with SALSA MLPA Probemix P021 SMA or SALSA MLPA Probemix P060 SMA Carrier using either DNA purified from peripheral blood or a crude extract from washed DBS cards, prepared as described in protocol C of section 7.2
Assay results are intended to be used in conjunction with other clinical and diagnostic findings, consistent with professional standards of practice, including confirmation by alternative methods, parental evaluation, clinical genetic evaluation, and counselling, as appropriate. The results of this test should be interpreted by a clinical molecular geneticist or equivalent.
This device is not intended to be used for standalone diagnostic purposes, pre-implantation or prenatal testing, carrier screening, or for the detection of, or screening for, acquired or somatic genetic aberrations.
1Please note that this kit is for in vitro diagnostic (IVD) use in the countries specified on the first page of this instructions for use. In all other countries, the product is for research use only (RUO).
2In people of African descent, the percentage of SMA patients with a homozygous exon 7 deletion may be lower (Labrum et al. 2007). This assay does not detect other causes of SMA such as pathogenic point mutations.
Disease
Spinal muscular atrophy (SMA) is a severe, recessive, neuromuscular disease for which treatment options are available. SMA is caused by a complete absence of functional copies of the SMN1 gene. In most populations, homozygous absence of the exon 7 DNA sequence of the SMN1 gene is observed in 95-98% of SMA patients. In most remaining cases, point mutations or partial deletions in the SMN1 gene are the cause of disease. For more information see Appendix 1: Background Information.
Assay
In the SALSA MC002 SMA Newborn Screen, PCR amplification of exon 7 of the SMN1 gene and the closely related SMN2 gene is performed, followed by fluorescent probe binding to the amplicons and generation of a melt curve (Figure 1, Strunk et al. 2019). Fluorescence is only measured during melt curve generation.
Absence of the SMN1-specific melt peak at 63°C is indicative of the absence of the SMN1 exon 7 DNA sequence. The presence of an SMN1 (63°C) and/or SMN2 (56°C) specific melt peak and an absent or low signal for the Q (quantity)-fragment specific melt peak (49°C) indicates successful assay performance and the use of sufficient sample DNA. The assay can be performed on a crude DNA extract prepared from a 1.5 mm or 3.2 mm punch of a DBS card or purified DNA from peripheral blood. More information on the assay can be found in Appendix 2: SALSA MC002 SMA Newborn Screen.
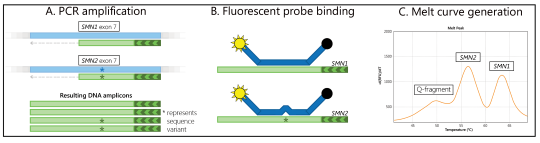
Figure 1. Summary of assay steps. (A) The exon 7 regions of SMN1 and SMN2 are amplified with a single set of primers, with one primer in excess. (B) A fluorescently-labelled probe binds to the amplicons. (C) The resulting melt curve indicates SNM1 and SMN2 sequence presence and if there was sufficient DNA used.
Download our demosheet for more information, read more about newborn screening for SMA on our website, or download a brochure about newborn screening for SMA.
Comparison of SMA products available from MRC Holland
MRC Holland offers four different assays for SMA that fit the complete range of genetic testing needs. The table below indicates which product can best be used for which purpose.
| Currently Viewing | |||||
|---|---|---|---|---|---|
| MC002 | P021 | P060 | P460 | ||
| Properties | CE-marked | yes | yes | yes | yes |
| Technique | Melt Assay | MLPA | MLPA | MLPA | |
| Used for | Neonatal Screening | ● | ○ | ○ | |
| Patient Detection | ● | ○ | ○ | ||
| Carrier Detection | ○ | ● | ● | ||
| Silent Carrier # Detection | ● | ||||
| Coverage | SMN1 exon 7 | ✓ Δ | ✓ | ✓ | ✓ |
| SMN1 exon 8 | ✓ | ✓ | ✓ | ||
| SMN2 exon 7 | ✓ Δ | ✓ | ✓ | ✓ | |
| SMN2 exon 8 | ✓ | ✓ | |||
| SMN1+2 exon 1-8 | ✓ | ||||
| Silent Carrier SNP Probes | ✓ | ||||
| Patient Detection Confirmation | P021; P060; P460 | MC002 * | MC002 | MC002 | |
| Product page | MC002 | P021 | P060 | P460 | |
● Primary test.
○ Secondary test.
# Increased detection of silent carriers: carriers with 2 SMN1 copies on one allele + 0 on the other.
Δ MC002: no absolute copy numbers aside from 0 determined.
* MC002 cannot detect exon 1-6 deletions.